

 Post Requirements
Post Requirements

 Post Requirements
Post Requirements
Product information
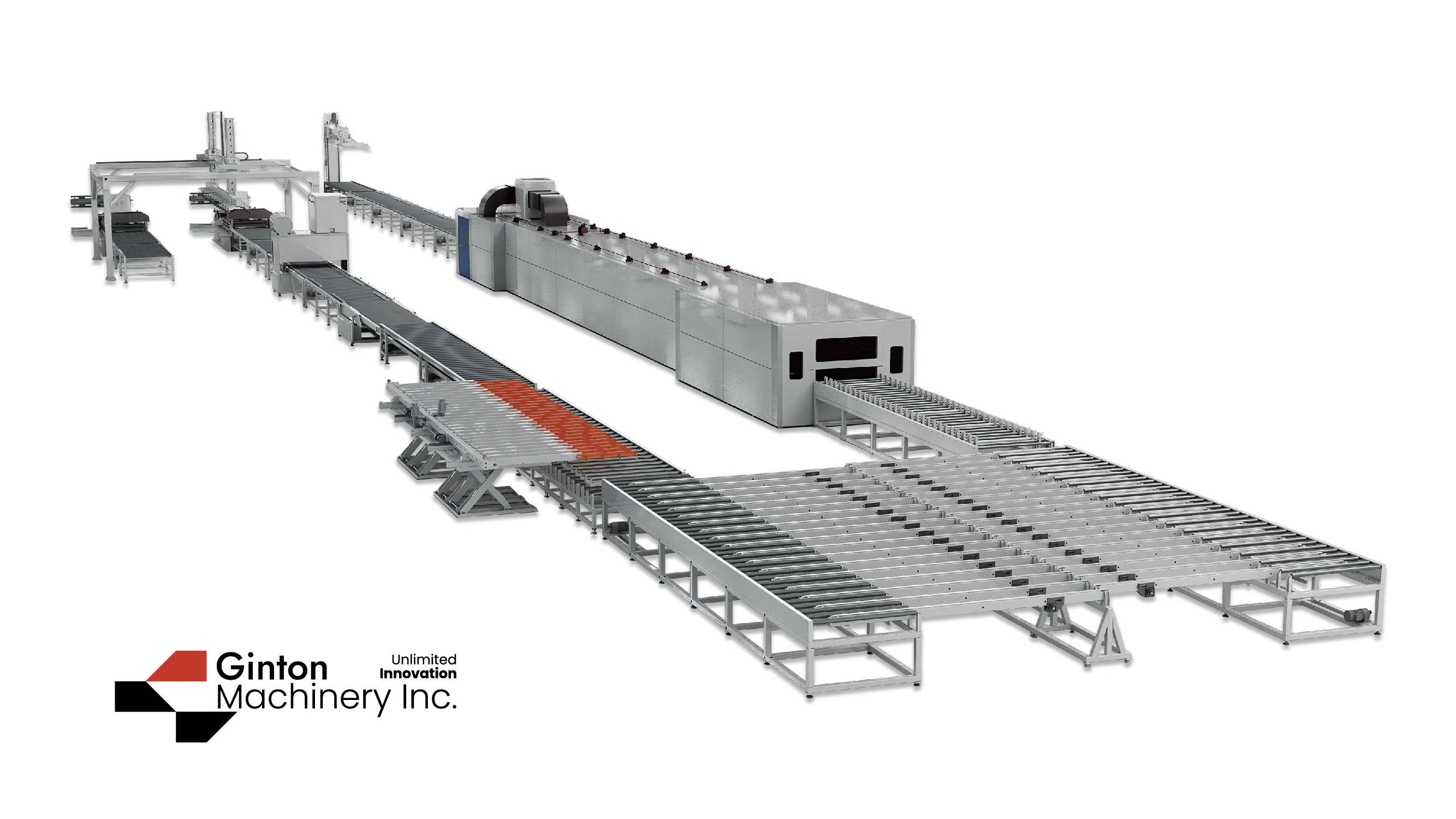
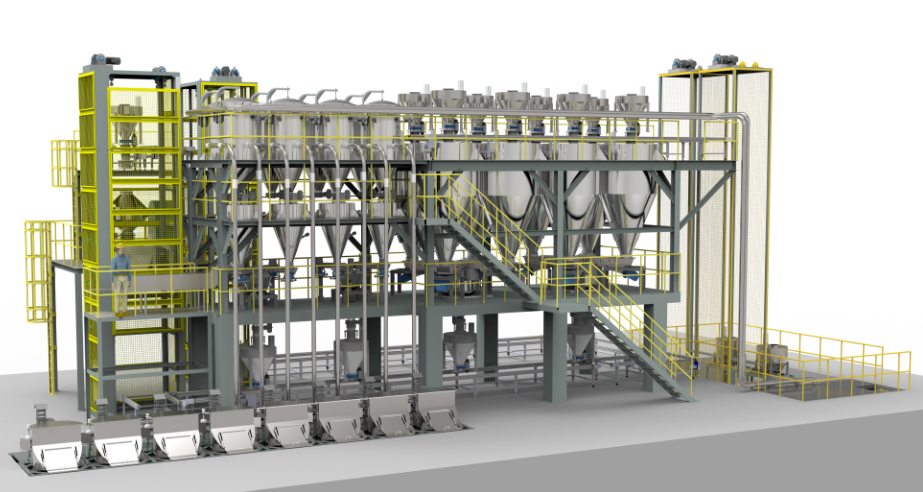
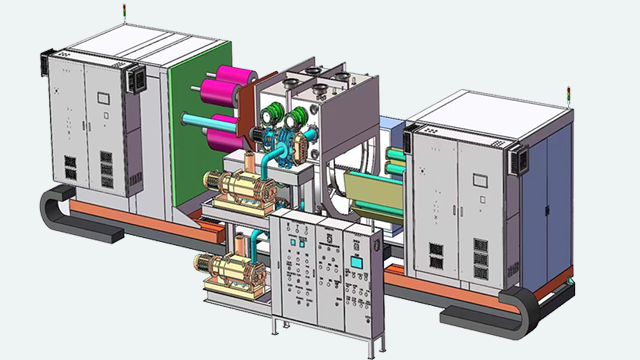
The company can undertake a variety of GMP packaging and palletization projects, and has successfully succeeded in multiple model customers.
1. The design and installation of the equipment meet the requirements of drug/food production and technology. It is safe, stable, reliable, easy to clean, disinfect or sterilize, facilitate production operations and maintenance, and prevent errors and cross -pollution.
2. The material selection of the device is strictly controlled. The components that are directly contacted with the material are non -toxic, corrosion resistance, and do not chemically change or adsorbed drugs with drugs.
3. Inner surface and working parts of the device that are directly contacted with the material, as much as possible, there are platforms, grooves and exposed bolt connections as much as possible. The surface is flat, smooth, without dead ends, easy to clean and disinfection.
4. Considering that the equipment should not pollute the environment outside the device, in view of the different conditions of each type of equipment, the whole machine takes measures such as dustproof, leak -proof, insulation, and noise prevention.
5. In the flammable and explosive environment, explosion -proof appliances are used and equipped with electrostatic and safety insurance devices.
6. When the trace foreign objects and lubrication generated by the driver's friction cannot be avoided, the device parts are implemented and isolate the studio. The lubricant used must not cause pollution to drugs and packaging containers. Observation and protection measures must be taken to enter the studio.
7. In addition to the general method of equipment cleaning, it is best to be equipped with local cleaning (CIP) to clean and sterilize systems of local sterilization (SIP).
8. Standardization, generalization, series and mechanical and electrical integration of equipment design. Realizing the continuous closed and automatic testing of the production process is the guarantee for the full implementation of the GMP requirements of the device.


















 Search
Search

